The Four Pillars Of
Nanotechnology Healing
 Healing Benefits
Healing Benefits
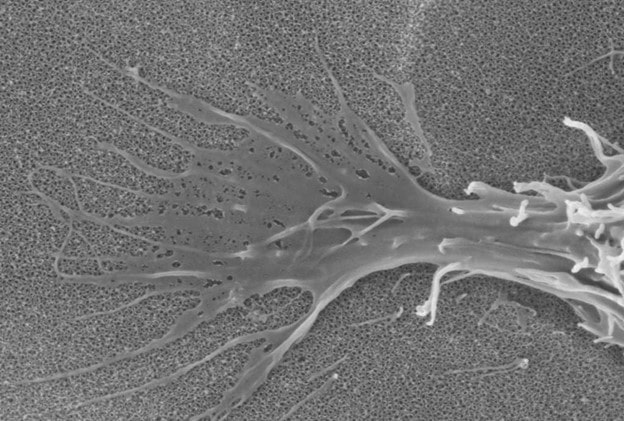
Nanotechnology is an engineered solution that addresses several overlapping issues.
These include bacterial colonization of the implant, inflammation around the implant, vascularization after implantation, and osseointegration. Solutions optimized for one of these problems may not solve others. We focused on innovating nanotechnology to address all four issues in a single approach.
Nanovis has successfully engineered our nanoVIS Ti™ Surface Technology to optimize implant integration from implantation to the end goal of osseointegration.
-
Nanovis' nanotechnology increases protein attachment for a lower immune response. It also decreases inflammatory cell attachment and activation while encouraging pro-healing macrophages.
-
Our surface technology reduces bacterial colonization and biofilm formation and spread.
-
Our nanotubes speed up growth on the implant, supporting new bone growth and accelerates healing.
-
Osseointegration is the marker of successful implantation. The sooner bone firmly attaches to the implant, the better the outcome.
Our Solution
Intellectual Property Protection
Nanovis® maintains a robust intellectual property portfolio consisting of several owned or licensed patents. All nanoVIS Ti™ Surface Technology components are protected by a series of patents. Additionally, the proprietary process used to produce our unique surface is safeguarded by multiple trade secrets.
Regulatory Strategy and Pathway
Nanovis® has achieved several significant regulatory milestones:
NanoVIS Ti™ Surface Technology is the only widely available platform nanotechnology to achieve FDA's nanotechnology designation.
We are the first company worldwide to obtain FDA clearance and nanotechnology designation for a pedicle screw system.
We are the second company worldwide to receive FDA clearance and nanotechnology designation for an interbody fusion cage.
The Master File for nanoVIS Ti™ Surface Technology has been referenced for multiple 510(k) clearances across two different product types in spine.
Clinical and Commercial Advantages
To validate the nanoVIS Ti™ Surface Technology, Nanovis® has commercialized a portfolio of spinal products. Learn more at nanovisspine.com.
Clinical Advantages
- FDA nanotechnology designation. First company in the world with designation on PEEK + Ti interbodies (CP Ti) and Open/MIS pedicle screws (ELI Ti).
- Statistically significant and superior comparative surface data in the label – hMSC mineralization and osteoblast mineralization at 21 days, in vitro.
Commercial Benefits
- On-label marketing for superior positioning and messaging.
- Superior FDA label used to drive new surgeon conversions, hospital approvals, and technology price premiums.
- Surface-on-a-surface story — add a nano surface to a macro-porous 3D-printed surface or a micron-roughened base.
- Surgeon engagement at a scientific and innovation level, enabling studies, papers, podium presentations, etc.

Let’s talk nano surface
Join Our Newsletter
We’ll send you a newsletter once a month, no spam.