What is Nanotechnology?
Nanotechnology is defined as the understanding and control of matter at dimensions of roughly 1 to 100 nanometers, a scale at which unique properties of materials emerge that can be used to develop novel technologies and products. At the nanoscale, the physical, chemical, and biological properties of materials differ from the properties of matter either at smaller scales, such as atoms, or at larger scales that we use in everyday life such as millimeters or inches. Nanotechnology involves imaging, measuring, modeling, and manipulating matter only a few nanometers in size.
nanoVIS Ti Surface Technology
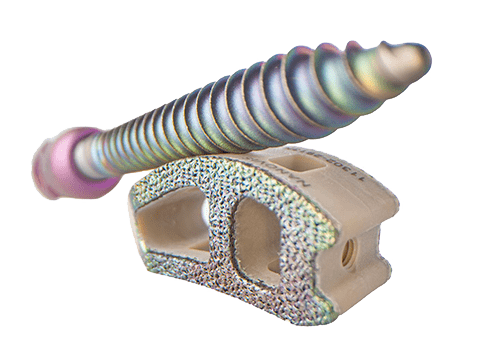
nanoVIS Ti Surface Technology, applicable to CP Ti and Ti alloy, is a bioceramic bonegrowth nanotube surface specifically designed to increase and accelerate biologic fixation.
IP & Clinical Validation
Nanovis® has defensible intellectual property with a patent portfolio consisting of 33 owned or licensed patents.
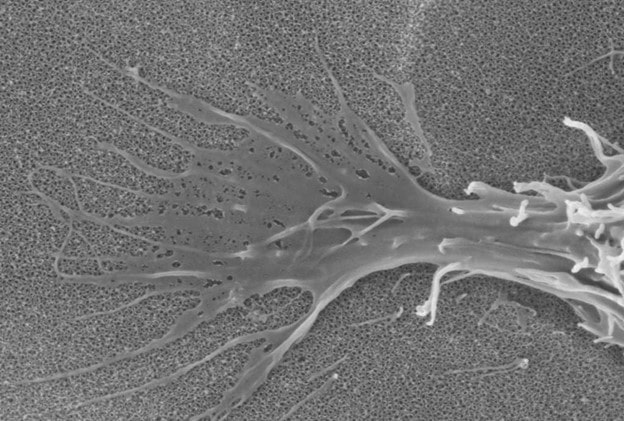
nanoVIS Ti Surface Technology possesses a controlled nano structured surface composed of nanotube arrays having an average pore size between 60-80 nanometers. These nanotube arrays have been shown to increase and accelerate calcified extracellular matrix production on human osteoblasts and human mesenchymal stem cells at 21 days, in vitro, compared to other surfaces commonly used in orthopedics.
nanoVIS Ti Surface Technology possesses a controlled nano structured surface composed of nanotube arrays having an average pore size between 60-80 nanometers. These nanotube arrays have been shown to increase and accelerate calcified extracellular matrix production on human osteoblasts and human mesenchymal stem cells at 21 days, in vitro, compared to other surfaces commonly used in orthopedics.
Regulatory Validation
nanoVIS Ti Surface Technology has demonstrated the elements to be considered nanotechnology as outlined in the FDA nanotechnology guidance document.
Nanovis was the first company in the world to receive FDA clearance and nanotechnology designation for Next Gen Ti + PEEK Surface Technology Interbodies (Nano FortiCore) and is the first and only company in the world to receive FDA clearance and nanotechnology designation for Open and Minimally Invasive Pedicle Screws (Nano FortiFix).
Nanovis was the first company in the world to receive FDA clearance and nanotechnology designation for Next Gen Ti + PEEK Surface Technology Interbodies (Nano FortiCore) and is the first and only company in the world to receive FDA clearance and nanotechnology designation for Open and Minimally Invasive Pedicle Screws (Nano FortiFix).
Operational Validation
The application of nanoVIS Ti Surface Technology is a validated manufacturing process and can happen in the production line. In addition, Nanovis’ production protocols call for sterile packaging of all nanotechnology products reducing the requirements for excessive field inventory and reducing the cleaning and safety risks from repeated sterilizations.
The application of nanoVIS Ti Surface Technology is both economical and efficient. Based on our research, applying the surface and subsequent sterile packaging, the cost could be 1/3 to 1/2 of the cost of applying alternative technologies like hydroxy appetite (“HA”) without the various clinical issues associated with HA removal.
The application of nanoVIS Ti Surface Technology is both economical and efficient. Based on our research, applying the surface and subsequent sterile packaging, the cost could be 1/3 to 1/2 of the cost of applying alternative technologies like hydroxy appetite (“HA”) without the various clinical issues associated with HA removal.

In addition to the Master File with product specific 510k clearance pathway and the first-ever FDA-cleared nanotechnology pedicle screw, Nanovis has world-class research and manufacturing facilities making them poised to scale.
Commercial Validation
nanoVIS Ti Surface Technology is currently cleared on seven implant systems in spine to include commercially pure titanium and titanium alloy implants. Nanovis has served over 10,000 patients with around 10,000 technology implants since 2015.
Nanovis has been recognized by Global Health and Pharma as the best nanotechnology driven implant company, and Med Tech Outlook as a Top 10 Orthopedic solution provider.
In an industry facing constant pricing pressures, vendor compression, and commoditization of implants, nanoVIS Ti Surface Technology has proven market share growth, price increases, hospital contract access, formulary carve outs, on label marketing campaigns, and surgeon and sales force excitement.
Please contact Nanovis at (877) 907-NANO
or Email info@nanovistechnology.com


Connect With Us
Columbia City, IN 46725
United States
Recent News