NANOVIS® Corporate News
This funding supports surging demand from surgeons and distributors for Nanovis’ nano-technology enhanced spinal implants.
Carmel, Ind. (Aug. 21, 2018) – Nanovis, an innovative and fast-growing technology company selling nano-technology enhanced spinal implants, announced today the successful completion of a $5.5 million funding round brokered by Commenda Securities. Key investors include Elevate Ventures, 1st Source Capital Corporation, Purdue’s Foundry Investment Fund, Commenda Capital, and Ellipsis Ventures.
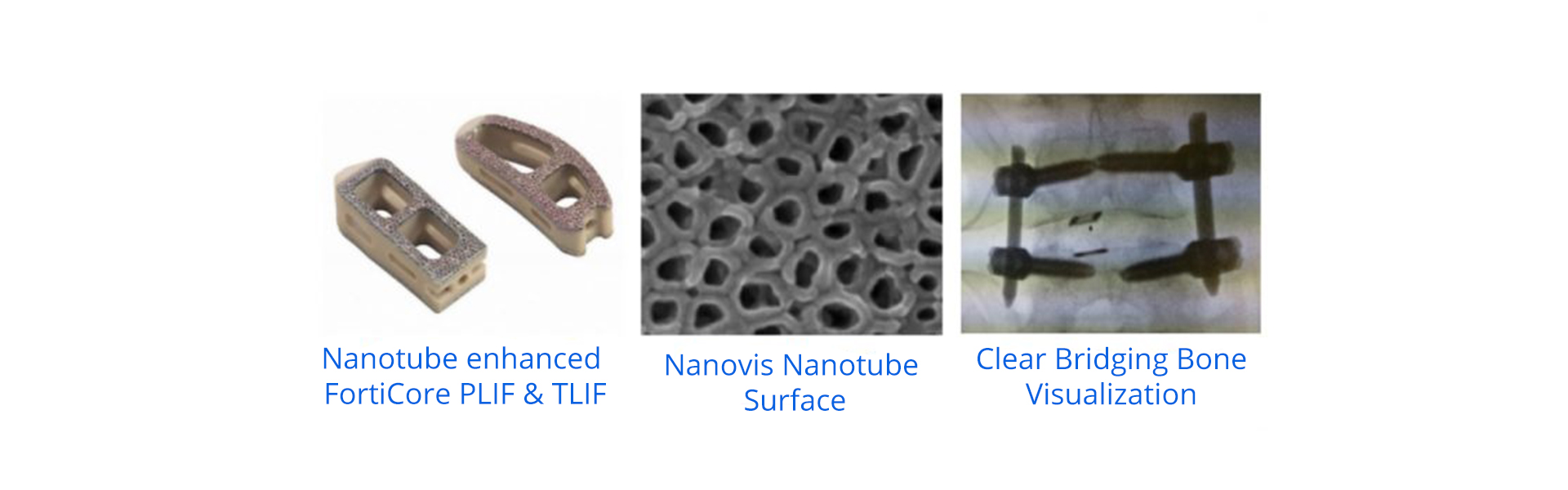
Spinal Implants Cleared with the Most Advanced Nanosurface and Best Imaging
Carmel, Ind. (March 28, 2018) – Nanovis, today announced the successful FDA clearance of its FortiCore® TLIF and PLIF interbodies featuring a Nanosurface-enhanced deeply porous titanium scaffold intermolded with a PEEK core.
Nanovis’ Deeply Porous Spinal Implants Show Favorable Clinical Adoption.
Carmel and Columbia City, Ind. (February 16, 2017) – Nanovis, a life sciences company committed to developing implant systems that reduce fixation related complications and infections, today announced the successful alpha launch of its FortiCore® PLIF featuring a deeply porous titanium scaffold interdigitated with a PEEK core, and implantation of the 2,000th FortiCore® implant.
Nanovis’ Deeply Porous Spinal Implants with Nanotube-Enhanced Surfaces May Significantly Improve Patient Recovery from Spinal Fusion Procedures
Carmel, Ind. (March 8, 2016) – Nanovis, a life sciences company committed to developing scientifically advanced regenerative platforms for implantable medical devices, today announced a grant award from the National Institute on Aging, part of the National Institutes of Health (NIH).
Proprietary Approach Layers Titanium Scaffold Surface Technology on PEEK Implants Creating Differentiated Devices
CARMEL, Ind., Oct. 15, 2015 /PRNewswire/ — Nanovis, a life sciences company committed to developing scientifically advanced regenerative platforms for implantable medical devices, today announced the availability of the FortiCore® wedge shaped lordotic cervical cage and a transforaminal lumbar interbody fusion (TLIF) device with increased scaffolding.
Combination of a deeply porous titanium scaffold with a PEEK center and advanced nanotechnology may reduce healing and fixation times
Carmel, Ind. (September 22, 2015) – Nanovis Spine today announced the award of a significant research grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH).
Carmel, Ind. (June 18, 2015) – Nanovis Spine, LLC (Nanovis) announced today the launch of the company’s FortiBridge® cervical plate system. FortiBridge cervical plates allow for high angulation screw placement with a smooth, esophagus-friendly profile. The FortiBridge cervical plate system offers a full range of short and long sizes in either steam sterilizable or individually sterile packaging formats.
Carmel, Ind. (September 22, ) – Nanovis Spine, LLC (Nanovis) announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of the company’s sterile packed FortiCore® interbody fusion devices. Unlike conventional implants comprising PEEK or sprayed titanium coating, FortiCore® implants have the benefits of a highly porous titanium scaffold engineered for strong, durable integration with a PEEK core.







Connect With Us
Columbia City, IN 46725
United States
Recent News